Abstract
Objective:
During tumor development, energy constraints caused by malnourished microenvironments could exert selective pressure on cancer cells. Tumor cells are driven to metabolic reprogramming to meet the increased demand for energy and metabolites for their rapid proliferation and survival. Chronic lymphocytic leukemia (CLL) is a disease with about 1% of CLL cells proliferating every day which is highly than commonly thought. CLL cells were reported to maintain high levels of proliferation through metabolic changes, but extensive studies did not clearly explain the underlying mechanism of driving genes in CLL metabolism. Circular RNA (circRNA) has recently been shown to play an important role in cell metabolism through lipid accumulation. The purpose of this study is to explore the role of circRNA in lipid metabolism of CLL and provide novel therapeutic targets for CLL.
Methods:
To analyze circRNAs expression profiles and metabolism map in CLL, peripheral blood mononuclear cells (PBMC) from 53 treatment-naïve CLL patients were collected for transcriptome sequencing. Candidate circRNA circRIC8B in a larger cohort of patients was validated and the clinical characteristics were analyzed. Overexpression and knockdown virus were constructed to infect CLL cells, and untargeted metabolomics was used to find the key lipid metabolic pathway modulating by circRIC8B. The oncogenic functions of circRIC8B were further measured in CLL cell lines (MEC-1 and JVM-3) by performing CCK8 assay, flow cytometry, nile red staining and triglyceride detection. Moreover, we explored the molecular mechanisms of circRIC8B and verified the interactions among circRIC8B, miR-199b-5p and LPL by performing RNA-FISH, RIP, dual-luciferase reporter assay and Western blotting. The killing effects of lipid metabolism inhibitors on CLL cells were detected by CCK8 and flow cytometry.
Results
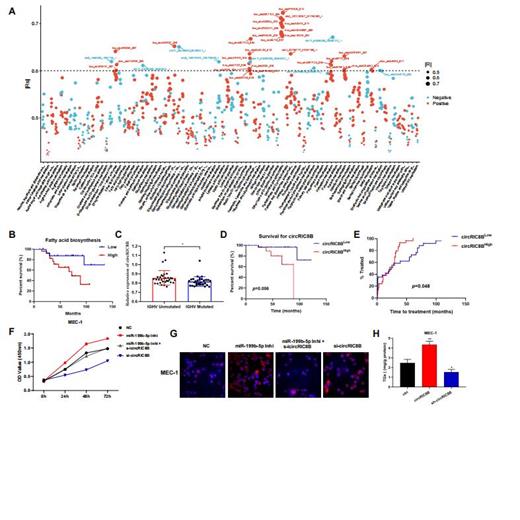
Transcriptome analysis showed that abnormal lipid metabolism was significantly related to the survival and prognosis of patients with CLL, and circRNAs could be involved in the regulation of lipid metabolism. Kaplan-Meier survival analysis confirmed that patients with higher fatty acid biosynthesis had a significantly lower OS (Figure 1A-B). circRIC8B which is positively correlated with the expression of lipoprotein lipase (LPL) was finally selected for further investigation. qRT-PCR analysis showed that circRIC8B was significantly higher expressed in CLL compared with healthy donors. Moreover, consistent with the sequencing results, circRIC8B was positively correlated with LPL and highly relevant to IGHV region mutation status, which has long been considered as an important prognostic indicator of CLL (Figure 1C). Patients with higher circRIC8B level are associated with worse survival and advanced disease progression (Figure 1D and E). LC-MS/MS results showed that circRIC8B are able to modulated lipid metabolism of CLL cells. Functional analysis demonstrated the promoting role of circRIC8B in cell proliferation. Nile red staining showed lipid accumulation in CLL cells with circRIC8B overexpression increased significantly, while lipid accumulation in circRIC8B knockdown cells decreased significantly, and the quantitative results of triglycerides were similar.
Next, we unraveled an original mechanism in CLL that up-regulated circRIC8B was mainly enriched in the cytoplasm, acted as a "sponge" of miR-199b-5p. CCK8 assay, nile red staining showed that the cell viability and lipid accumulating of CLL cell lines were evidently decreased after RNAi of circRIC8B and this result could be reversed by miR-199b-5p inhibitor transfection (Figure 1F-H). In addition, ezetimibe, one of the inhibitors of lipid metabolism was found effectively inhibit the proliferation and promote apoptosis of CLL cells.
Conclusions
In conclusion, as an independent prognostic factor of CLL, circRIC8B was involved in the progress of CLL disease through the miR-199b-5p/LPL axis. In addition, circRIC8B is a key factor in regulating lipid accumulation in CLL, resulting in significant changes in cellular lipid storage, thus supporting the proliferation of CLL cells. Metabolic inhibitor Ezetimibe can effectively block this process and exert anti-tumor functions. This study provides new clues for the role of circRNA in abnormal lipid metabolism of CLL and novel therapeutic strategy for CLL patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal